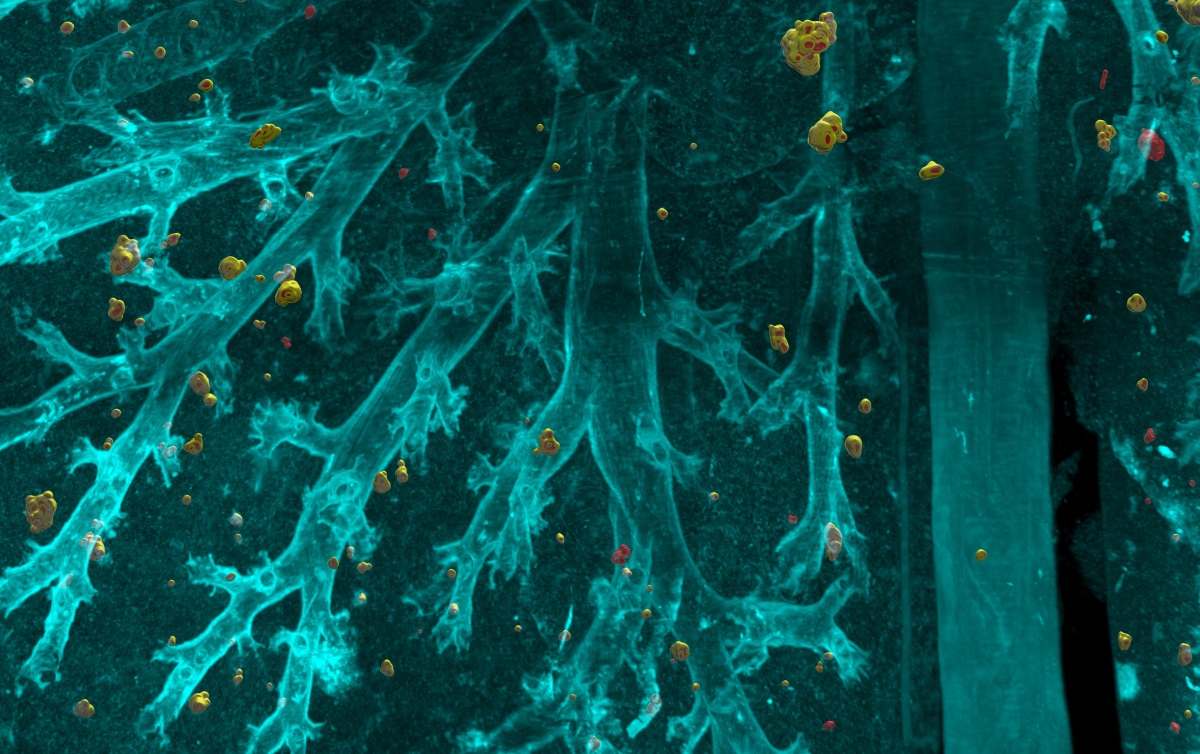
Despite recent medical advances, cancer remains one of the leading causes of death worldwide. More than 90% of cancer-related deaths are from metastases rather than from the primary cancerous growth. Such metastases develop when cancer cells spread to new areas of the body whilst evading the body’s immune system.
Owing to the limited resolution of preclinical imaging techniques such as bioluminescence imaging and MRI, comprehensive detection of small metastatic lesions in the bodies of model animals has not been possible. These shortcomings have severely hampered the development of effective treatments by assessing the efficacy of new drug candidates, and resulted in a lack of knowledge about spreading mechanisms of diverse cancer types.
To overcome the obstacle of detecting cancer metastasis, researchers at Helmholtz Zentrum München, the Ludwig Maximilian University of Munich (LMU) and the Technical University of Munich (TUM) have developed a novel deep-learning based algorithm called DeepMACT (deep learning-based metastasis analysis in cleared tissue). DeepMACT enables automated detection and visualization of even the smallest of metastases and can also determine whether a drug has reached them.
The image-based AI algorithm
The researchers used a tissue clearing method called vDISCO to render the complete mouse body transparent. Using laser-scanning microscopes, they imaged the transparent mouse in 3D, allowing visualization of the smallest metastases, down to individual cancer cells. Manual analysis of such high-resolution images is time-consuming, however. Hence, DeepMACT was born (Cell 10.1016/j.cell.2019.11.013).
DeepMACT enabled the team to visualize exactly which cancer metastases were targeted by a drug candidate and which ones were missed. Their analyses showed that antibody-based drugs – for example, an antibody named 6A10, which is among the most effective treatments available – can miss up to 23% of the metastases in the bodies of affected mice. In addition, the team used this method to analyse metastatic spread in lung, breast and pancreatic cancer, and to observe how the metastases spread through the body at different time points.
“DeepMACT is the first method to enable the quantitative analysis of metastatic process at a full-body scale,” adds first author Chenchen Pan.
In detecting the metastases, the DeepMACT method not only matched the performance of manual detection but completed the analysis 300 times faster. “With a few clicks only, DeepMACT can do the manual detection work of months in less than an hour,” says Oliver Schoppe, a doctoral candidate at TUM.
As DeepMACT is publicly available and easily adoptable, the scientists are hopeful that the technology will be used by other laboratories involved in diverse tumour research and treatment options. Today, only about 5% of new drug candidates are successful in cancer treatment. Application of DeepMACT in pre-clinical research could improve the identification and development of better drug candidates for clinical trials. This approach should significantly increase the success rate of cancer drug candidates, improve the drug development process and potentially save many more lives.