The idea of repairing the brain by replacing the neurons that die in Parkinson’s disease has been a long-standing dream for researchers in the field. Over the past few decades, many cell types have been put forward as candidates to replace dying neurons1, including cells derived from the midbrain of human fetuses. But the focus has now turned to pluripotent stem cells, which can give rise to almost any cell type. Transplantation of specific neuron progenitors derived from these stem cells is on the verge of being tested in the clinic2, but limitations remain, including low rates of survival and suboptimal function of the transplanted neurons3. Writing in Cell Stem Cell, Gantner et al.4 address some of these limitations.
Parkinson’s disease is caused by the degeneration of a population of neurons that reside in the brain’s substantia nigra and project to the striatum, where they release the neurotransmitter molecule dopamine. A plethora of growth factors are involved in the development of these dopaminergic neurons in the immature brain, but these factors are either lacking or expressed at much lower levels in the adult brain. The introduction of growth factors into the adult brain, therefore, might be a way to improve the success of cell-engraftment approaches.
Gantner et al. set out to investigate the possibility of using one such factor, glial-cell-line-derived neurotrophic factor (GDNF). This type of approach has a long history. Several clinical studies have analysed whether the administration of GDNF to the brain could improve the outlook for people with Parkinson’s disease (although these attempts have met with limited success5). And work has indicated that GDNF can enhance the survival and function of grafts of fetal dopaminergic neurons6.
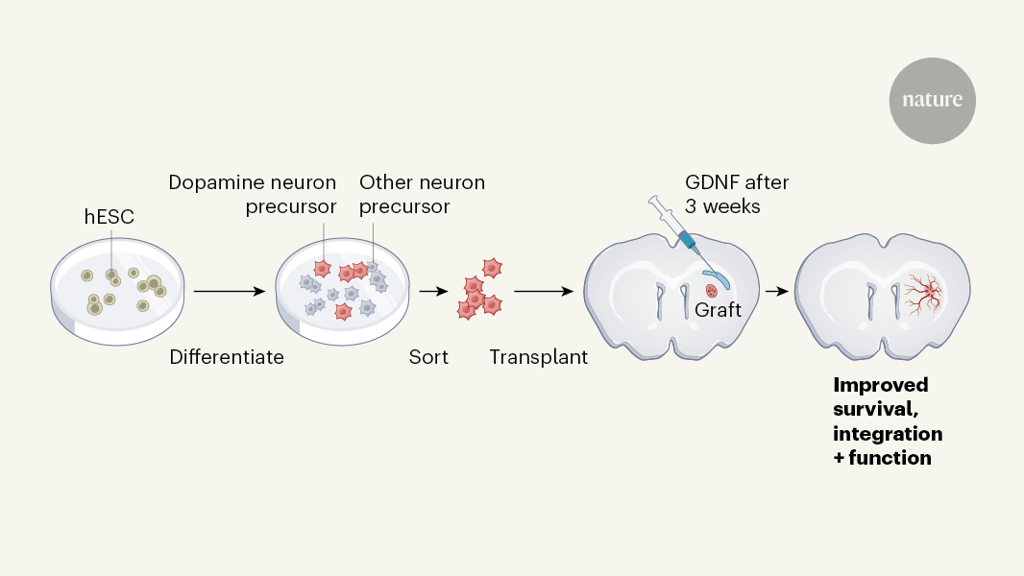
Gantner and colleagues used a type of pluripotent stem cell called human embryonic stem cells. They engineered the cells to fluoresce when they expressed either a protein specific to midbrain dopaminergic neurons, called PITX3, or a protein specific to dopaminergic-neuron progenitors, called LMX1A. This approach allowed the researchers to easily quantify the number of dopaminergic neurons and precursors both in vitro and in brain grafts. The group used an established protocol to convert the stem cells to dopaminergic-neuron progenitors in vitro, before transplanting them into the brains of mice or rats lacking dopaminergic neurons in the midbrain (Fig. 1). They then assessed how GDNF (injected into the striatum in a viral vector) affected the survival of the grafted dopaminergic neurons and their integration into the rodent brain.
The authors delivered GDNF one week before or three weeks after transplantation — time points selected to capture the effects of GDNF on the initial survival of the transplanted cells or on later stages of neuron maturation and integration. They found that GDNF delivery increased neuron survival at both time points, although more so if it was delivered before the transplant. However, several lines of evidence indicated that only late GDNF delivery improved functional integration into the brain — this timing resulted in increased density of neuronal fibres in the striatum, increased synaptic connections between grafted neurons and host neurons, and improvements in motor coordination.
The work, therefore, demonstrates that it is crucial to understand the biology of GDNF action at each stage of dopaminergic-neuron development if this type of treatment is to be beneficial. It also suggests that GDNF treatment could be used to improve the next wave of stem-cell-based neuron transplants. It is likely that the optimal timing of GDNF delivery in humans would be comparable, because the timing of survival and maturation of transplanted human neurons is remarkably similar when grafted into a mouse, rat or primate brain7.
In addition to problems with neuronal integration, current approaches to stem-cell differentiation result in a mixed cell population from which only a relatively low percentage of grafted cells become dopaminergic neurons. For example, six months after Gantner et al. transplanted unsorted dopaminergic-neuron precursors into the rodents, less than 1% of cells in the graft were dopaminergic neurons — even in the presence of GDNF. The authors took advantage of the fact that their cells carried fluorescent reporters to address this point, by purifying the cells before transplantation and engrafting only those that expressed LMX1A. This approach greatly improved the percentage of dopaminergic neurons in the graft, to 10% of total cells after six months.
Gantner and colleagues’ experiments point to ways to improve the efficiency of transplant approaches to treat Parkinson’s disease. However, they also highlight further issues that need to be overcome. For instance, in unsorted grafts, GDNF treatment increased overall cell numbers (and therefore the number of cells of unwanted types), highlighting that improvements in cell survival alone are not enough. The fluorescence-based sorting approach used by the authors would present considerable manufacturing and scalability challenges in the clinic. However, drug-based or magnetic sorting strategies might be appropriate alternatives. In addition, it will be important to demonstrate the long-term safety of combining grafting and sustained GDNF expression, ideally in primates, before testing the authors’ approach in people.
Another major question going forward — both for approaches to treat Parkinson’s disease and for regenerative therapies more broadly — is whether generating the ‘authentic’ cell that is lost in disease is sufficient, or whether genetically engineering cells could enhance the benefit of transplants. The cell-lineage reporters used by Gantner and colleagues are just one example of the second approach. Another prominent idea is the development of genetically engineered universal donor cells8,9. Currently, many cell-based therapies use donor cells, in which case patients must receive immunosuppressive treatments to prevent graft rejection. A universal donor cell engineered to lack surface antigen proteins that can be recognized by the immune system would prevent this issue.
For Parkinson’s disease specifically, cells engineered to express lower-than-normal levels of the disease-associated protein α-synuclein are of interest, because this approach might prevent disease spreading into the graft10. Finally, methods to genetically control graft activity or connectivity with host cells could help to fine-tune graft function according to the individual needs of the patient, whose disease condition might be changing over time11.
The increasingly routine nature of genetic engineering in pluripotent stem cells offers an exciting opportunity for regenerative medicine — being able to generate any cell type expressing any therapeutic gene product on demand. However, to take advantage of such technology, it is paramount to understand the biology of regeneration. Taking another look at molecules such as GDNF is a timely advance that could give neuronal grafts some extra help, optimizing therapeutic success.